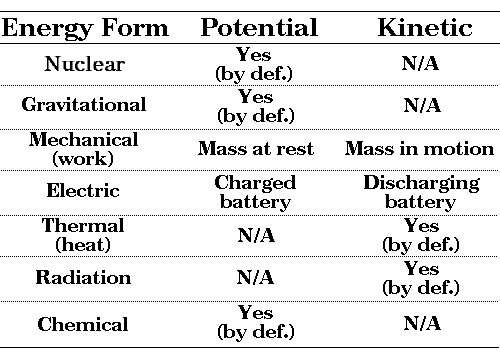
Here is the summary of the most important energy forms. Some exist in both their potential version (energy at rest, waiting to be used) and their kinetic version (energy in motion); others exist only either as potential or kinetic energy.

From the standpoint of energy quantity, all is well. In all energy conversions, energy is conserved; no energy is lost. This is best illustrated by an idealized pendulum in which there is 100% interconversion between potential and kinetic energy. (In a real-world pendulum, not as much kinetic energy is obtained; part of the potential energy is converted to heat, because of friction, but energy is still conserved.)
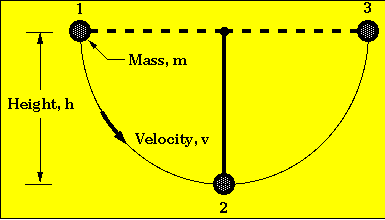
In position 1 all the energy is potential, in position 2 all potential energy is converted to kinetic energy, and in position 3 all the kinetic energy is converted to potential energy.
From the standpoint of energy quality, however, some energy conversions are 'better' than others. By this we mean that nature imposes a lower 'tax' on certain energy conversions and a higher 'tax' on other energy conversions. The most important example of this fact of life is the interconversion between work and heat.

Nature has a way of 'expressing displeasure' when humans attempt to 'go against it'. It imposes a greater 'tax' on conversion of heat to work than on conversion of work to heat. Much less useful energy is typically obtained in conversion of heat to work than in conversion of work to heat.
Heat (or thermal energy) is the consequence of random (disordered) motion of atoms and molecules in a substance; therefore, it is a form of energy that has a high entropy. In contrast, work (or mechanical energy) is the consequence of ordered (e.g., unidirectional) motion of substances; therefore, it is a form of energy that has a low entropy. Nature 'likes' energy conversions that result in an entropy increase. A full understanding of this fact of life requires the introduction of several concepts from the field of thermodynamics. Let's not get into thermodynamics now. Let's just accept this fact and let's explore some of its implications.
This is bad news, because our industrialized society relies so much on conversion of heat to work. In a typical electric power plant, only about 35% of the chemical energy of coal is converted to electricity. This is because the chemical energy is first converted to heat, when coal is burnt, and the heat is then converted to work and ultimately to electricity. In a typical automobile engine, only about 25% of the chemical energy of gasoline is converted to mechanical energy of the rotating wheels. So 75 cents of every dollar that we spend on gasoline is essentially wasted.
Even in nuclear power plants the conversion of nuclear energy to electricity is constrained by this 'thermodynamic bottleneck'. Only when we really learn how to harness solar energy (hopefully some time early in the 21st century) will we bein a position, at least in principle, to avoid paying this high tax to Mother Nature. The efficiency of conversion of solar energy to electricity does not have the thermodynamic constraint that plagues the conversion of heat to work.
Energy: qualitative definition
Energy: quantitative definition
Energy conversion efficiency
Go back to MatSc 101 home page