
It is with increasing frequency that we read articles such as this one (TIME, October 2, 1995):

Natural gas is mostly methane, CH4, whose molecular weight is 16 and which has a heating value of 24000 BTU per pound. When methane burns, it combines with oxygen in air to produce carbon dioxide and water. From elementary chemistry, we know that one mole of methane (containing 6x10^23 molecules) combines with one mole of oxygen to produce one mole of carbon dioxide. Therefore, for every 16 pounds of methane, 44 pounds of carbon dioxide are produced. Thus, the amount of carbon dioxide produced per BTU of energy generated by burning natural gas is obtained as follows:

A low-rank coal, lignite (with a heating value of approximately 7000 BTU/lb), can be represented simply as follows: for every 100 carbon atoms in coal, there are 83 atoms of hydrogen, 23 atoms of oxygen and 1 atom of nitrogen. In other words, it contains (by weight) 72% carbon, 22% oxygen, 5% hydrogen and 1% nitrogen. Its greenhouse effect is obtained as follows:
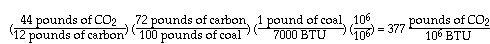
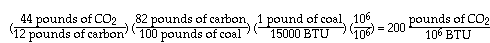
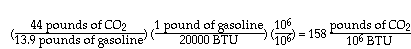
The other bad news is also illustrated above. Low-rank coals, which are abundant in the U.S., are increasingly attractive for power plants because they contain less sulfur, and therefore cause much less acid rain, than typical bituminous coals. But we see that their combustion produces much more CO2 than the combustion of high-rank coals.
This is why the TIME article mentions turning to nuclear and solar energy, as a second recommended strategy to avoid the 'apocalypse'. Turning to nuclear energy is more easily said than done, because it opens another 'can of worms'. Turning to solar energy is of course environmentally desirable, but quite a few technical and economic problems need to be resolved before this can be a viable alternative in the near future.
Here is the most recent update, based on the important speech of President Clinton to the National Geographic Society, in preparation for the Earth Summit in Kyoto, Japan (December 1997).