What is Petroleum?
 Petroleum
is a naturally occurring, flammable liquid found within rock formations in
the earth, and is comprised of a mixture of hydrocarbons and other organic
compounds. The hydrocarbons, although varying widely depending on location,
are mainly alkanes (or “paraffins”), cycloalkanes, and various aromatic
hydrocarbons. The organic compounds may contain nitrogen, oxygen, and
sulfur, in addition to trace amounts of metals, such as iron. Petroleum
is a naturally occurring, flammable liquid found within rock formations in
the earth, and is comprised of a mixture of hydrocarbons and other organic
compounds. The hydrocarbons, although varying widely depending on location,
are mainly alkanes (or “paraffins”), cycloalkanes, and various aromatic
hydrocarbons. The organic compounds may contain nitrogen, oxygen, and
sulfur, in addition to trace amounts of metals, such as iron.
Petroleum
(like coal) is formed, in general, through the compression and heating of
organic materials over geologic time. Contrary to coal, which owes its
origins to the decomposition of terrestrial plant life, petroleum can be
traced to the burial of marine (and also lake dwelling) organisms,
primarily prehistoric zooplankton (protozoans, some types of copepods,
worms, krill, crabs, jellyfish, and the larvae of fish and other
invertebrates) and algae.
In other
words, we can think of oil (or gas or coal) as organic material that is
prevented from complete decay. Or, to think of it another way, the rotting
tree in your backyard will not turn into a lump of coal; but bury it
beneath a swamp, wait a few million years, and you might have a shot.
But,
we’re getting ahead of ourselves. To make the carbon compounds that will
one day fuel our cars and heat our homes, we need a more basic constituent
and, as we’ll see, that constituent gets its energy from the sun.
Photosynthesis: The influx of
energy
 Nearly all energy that we utilize on
earth is directly or indirectly a result of sunlight. Wind energy, for example,
is merely the result of convective atmospheric circulation caused by uneven
heating of the earth by the sun’s rays. There are exceptions, such as geothermal
energy (energy from the interior heat of the earth) and nuclear energy
(energy that is stored within the structure of atoms); although one could
certainly argue reliance between these energies (the sun (nuclear fusion),
and even the earth (radioactive decay), utilize nuclear energy, for
example). Fossil fuels are no exception; they gain their energy (albeit,
over extremely long periods of time) from the sun’s participation in
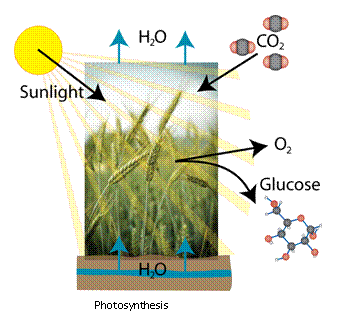
photosynthesis. The general idea of photosynthesis is as follows: Hydrogen
(from water) combines with carbon (from carbon dioxide) using energy from
the sun to form organic matter (glucose) and free oxygen: Nearly all energy that we utilize on
earth is directly or indirectly a result of sunlight. Wind energy, for example,
is merely the result of convective atmospheric circulation caused by uneven
heating of the earth by the sun’s rays. There are exceptions, such as geothermal
energy (energy from the interior heat of the earth) and nuclear energy
(energy that is stored within the structure of atoms); although one could
certainly argue reliance between these energies (the sun (nuclear fusion),
and even the earth (radioactive decay), utilize nuclear energy, for
example). Fossil fuels are no exception; they gain their energy (albeit,
over extremely long periods of time) from the sun’s participation in
photosynthesis. The general idea of photosynthesis is as follows: Hydrogen
(from water) combines with carbon (from carbon dioxide) using energy from
the sun to form organic matter (glucose) and free oxygen:
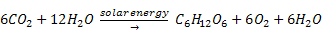
You may recall
that chlorophyll plays a role in this reaction, and you are correct.
Chlorophyll is the party responsible for absorbing the correct wavelength
of sunlight, which then allows the photosynthesis reaction to take place.
The
glucose from this reaction is then synthesized by autotrophic organisms
(those that get their energy from the sun) to construct all of the organic
matter that they need. This is the basic process that produces all of the
organic matter on earth. Primitive autotrophic organisms such as bacteria
and algae were among the first to accomplish this on our planet. In our
oceans, phytoplankton are the sunlight gathering autotrophic organisms that
do this, thus starting marine the food chain, and they inspire the course
of events that will eventually lead us to petroleum. But first, let’s build
an organism…
|