Refining
the oil shale
 In the
same manner that natural mineral catalysts help to transform kerogen to
crude oil through the process of catagenesis, metal catalysts can help transform
large hydrocarbons into smaller ones. The modern form of “catalytic
cracking” utilizes hydrogen as catalyst, and is thus termed
“hydrocracking”. This is a primary process used in modern petroleum
refining to form more valuable lighter fuels from heavier ones. In the
same manner that natural mineral catalysts help to transform kerogen to
crude oil through the process of catagenesis, metal catalysts can help transform
large hydrocarbons into smaller ones. The modern form of “catalytic
cracking” utilizes hydrogen as catalyst, and is thus termed
“hydrocracking”. This is a primary process used in modern petroleum
refining to form more valuable lighter fuels from heavier ones.
Oil that
is produced from the refining of oil shale is typically referred to as
synthetic crude oil, but the process is more closely allied with
traditional crude oil refining than syn-fuel processes such as “gas to
liquids”. The primary process is referred to as “retorting”, and involves
the “cracking” (or destructive distillation or pyrolysis) of larger carbon chains into smaller ones in the
absence of oxygen. Syn-fuel processes (such as Fisher-Tropsch) actually
build up larger hydrocarbons from smaller ones, which is the opposite of
cracking. Retorting is the cracking process used in shale oil refining, and
first breaks down the kerogen to release hydrocarbons, and then further
cracks the hydrocarbons into lower weight products.
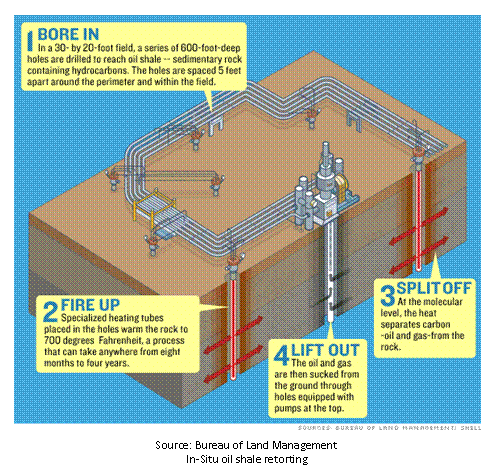
Retorting
can occur in a traditional refining capacity, or may be conducted in-situ. In-situ processes require that the oil shale be heated, to
release the petroleum liquids, prior to extraction from the ground.
Oil
shale distillates (products of retorting) typically favor the production of
middle-distillates (diesel and kerosene), and have higher concentrations of
nitrogen than crude oil. To produce light-distillates (such as gasoline)
additional processing, such as hydrocracking, is required to break down the
larger hydrocarbons. Also, the nitrogen must be removed through some
hydrotreating process, comparable to hydrogen desulfurization to remove
sulfur from crude oil, such as hydrodenitrogenation.
Because
hydrogen will be required in the refining process (bitumen is carbon rich
and hydrogen poor), an additional source of hydrogen (such as methane) is
needed. Therefore, some other fuel in addition to bitumen is needed to
produce synthetic crude oil, which adds to the cost and energy intensity
(and carbon dioxide emissions) of the process (2 fuels  1
fuel). 1
fuel). |